The United States has given final approval to Covid-19 injections for children aged 5 to 11.
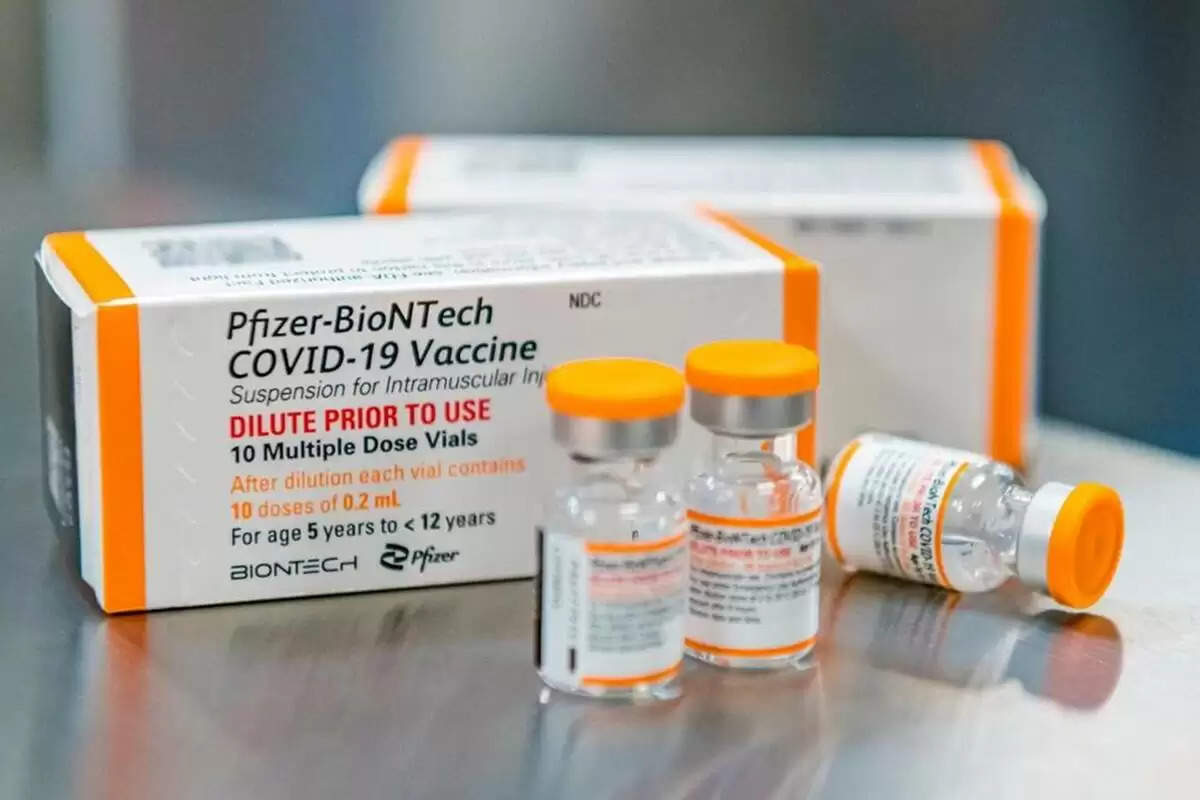
Pfizer's kid-size COVID-19 shot received final approval from US health officials on Tuesday, paving the way for a huge extension of the country's immunization campaign to include children as young as five years old.
The shots have previously been approved by the Food and Drug Administration for children aged 5 to 11 years old, with doses that are only a third of those given to teenagers and adults. The Centers for Disease Control and Prevention, on the other hand, makes explicit recommendations on who should receive FDA-approved vaccines.
Dr. Rochelle Walensky, director of the CDC, made the news just hours after an advisory council unanimously recommended that Pfizer's vaccines should be made available to the 28 million children in that age bracket.
The decision represents the first time that Americans under the age of 12 will be able to receive the powerful protection of any COVID-19 vaccine.
"As a mother, I encourage parents who have reservations about the vaccination to speak with their pediatrician, school nurse, or local pharmacist to learn more about the vaccine and the significance of getting their children vaccinated," Walensky said in a statement Tuesday night.
She added earlier in the day that while the risk of severe disease and mortality in young children is lower than in adults, it is nevertheless present — and that COVID-19 has had a profound social, mental health, and educational impact on children, including growing learning inequities.
"There are kids in second grade who have never had a regular school year," Walensky added. "Vaccination for children has the potential to revolutionize all of that."
The decision was dubbed "a watershed moment" by Vice President Joe Biden.
"It will help parents to stop worrying about their children for months," he said in a statement. "It will also minimize the amount to which children disseminate the illness to others." "It's a significant step forward in our fight against the virus for our country."
The American Academy of Pediatrics applauded the decision as its members prepare to administer the first shots into children's arms, which the CDC said might start "as soon as possible." The vaccine, developed by Pfizer and its partner BioNTech, will be given in two modest doses three weeks apart, on the same timetable as everyone else, but with a tiny needle.
Pfizer began distributing millions of the pediatric doses to states, doctors' offices, and pharmacies over the weekend, with orange caps to minimize confusion with adult vaccine vials with purple tops.
Many parents have pushed for vaccine protection for children so that they can resume regular childhood activities without jeopardizing their own health or possibly infecting a more vulnerable family member. However, CDC counselors acknowledge that many parents have doubts and maybe scared about the vaccine due to widespread misconceptions.
Members of the advisory group said they want parents to ask questions about the shots and understand that they're significantly better than taking a chance on their child avoiding a serious coronavirus infection. In terms of safety, more than 106 million Americans have received two doses of Pfizer's full-strength vaccines without complications, including more than 7 million 12- to 15-year-olds.
"I have vaccinated my children," said CDC expert Dr. Helen Keipp Talbot of Vanderbilt University, adding that she wouldn't advocate something to other families unless she was confident in it for her own. "We have witnessed the disease's damage."
According to government data, there have been more than 8,300 coronavirus-related hospitalizations of children aged 5 to 11, with roughly a third requiring intensive care. At least 94 deaths in that age category have been reported to the CDC, with more reports under investigation.
According to Pfizer's study of 2,268 children, the kid-size vaccine is approximately 91 percent effective at preventing symptomatic COVID-19, based on 16 diagnoses among kids who received dummy shots vs only three illnesses among kids who received the real vaccine.
The FDA looked at a total of 3,100 youngsters who had been vaccinated before finding that the doses are safe. After smaller doses, the younger children had equivalent or fewer responses, such as aching arms, fever, or achiness, than teens or young adults.
The sample size was insufficient to detect exceedingly unusual side effects, such as heart inflammation, which can develop after the second full-strength dose in young men and teen boys. Ultimately, regulators judged that the benefits of vaccination exceed the danger that younger children receiving a smaller dosage would also have that unusual harm.
Some CDC consultants believe that for some parents, the decision to vaccinate their children may be based on that small but frightening danger.
Dr. Matthew Oster, a pediatric cardiologist at Emory University, told the panel, "The probability of some form of serious heart involvement is substantially higher if you have COVID than if you get this vaccine."
What about the younger ones? Pfizer is currently studying vaccines for newborns and preschoolers, with results expected before the end of the year. The Moderna vaccine, which is manufactured in a similar way, is also being tested on young children. However, the FDA has yet to approve its use in teenagers, and the business is delaying its application for younger children while the agency conducts its review.
Other COVID-19 vaccines are being used in children under the age of 12 in a few countries, including China, which has just started vaccinations for 3-year-olds. However, many users of the Pfizer-BioNTech vaccine are keeping an eye on the US decision, and European regulators have only recently begun to consider the firms' child-size doses.
.png)